Protein Nuclear Magnetic Resonance (NMR) Service
Online InquiryIn life, proteins are constantly moving, so static three-dimensional structures are often not enough to explain their biological functions. To truly elucidate the structural basis for protein functions, it is necessary to study the "dynamic structure" of protein molecules. One of the main features of liquid phase nuclear magnetic resonance (NMR) technology is the ability to study the three-dimensional structure of proteins in a state closer to the physiological environment (pH, salt concentration, temperature, etc.). This technique can be used to study the dynamics of protein multiple sites at the atomic level through the nucleus relaxation process.
Creative Proteomics has an NMR platform that can provide customers with protein structure analysis services. We have Bruker AVANCEIII HD 800MHz and Bruker AVANCE III HD 600MHz two liquid nuclear magnetic resonance spectrometers, which can provide you with one-stop protein structure determination services, including protein expression, protein purification, NMR data collection, data molecule and protein structure analysis.
The Process of Protein Nuclear Magnetic Resonance (NMR) Service
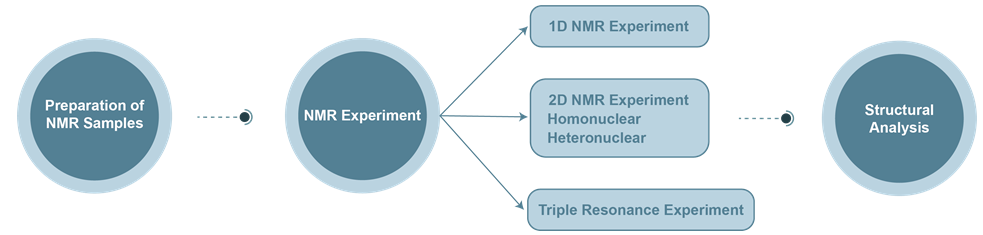
*The NMR spectrum of large molecular weight biomacromolecules is very complex, so this technique is often used to determine smaller molecular weight biomolecules.
Technology Platform
Bruker AVANCEIII HD 800MHz and Bruker AVANCE III HD 600MHz two liquid nuclear magnetic resonance spectrometers are available.
We Provide the Following Services, including but not Limited to:
- To study the three-dimensional structural basis of protein-protein interactions: Protein interactions in cells are dynamic and low-affinity. And many complexes are transient and unstable. For such dynamic complexes, it is difficult to solve the crystal structure, and nuclear magnetic resonance spectroscopy is particularly suitable for studying transient, dynamic complexes.
a. Study the three-dimensional structure and function of biological macromolecules and their complexes in solution
b. Study dynamic interactions between biological macromolecules and ligands
c. Study protein-protein interactions in living cells
- Kinetic study of proteins: A protein is a dynamic system in which residues are coupled to each other. Ligand binding causes signals to be transmitted inside the protein, which causes allosteric effects. NMR is particularly suitable for studying the dynamics of proteins. The obtained kinetic information can be specifically determined to the group under study. The spin-lattice relaxation time, spin-spin relaxation time, and NOE measurements can be used to obtain picosecond-nanosecond kinetic information.
- Study protein folding and unfolding: The study of unstructured proteins helps to understand protein folding, protein aggregation, and protein fibrosis. Structurally unstructured proteins are associated with diseases of amyloid fibrosis such as Prion's disease and Parkinson's disease. Some endogenous unstructured proteins undergo a disorderly transition to an ordered structure during protein binding. NMR can provide unstructured or partially structured proteins, as well as many aspects of the protein folding process.
- For drug screening and design: Many drugs target on membrane proteins. Creative Proteomics provides solid-state NMR or liquid NMR techniques to study the structure and function of membrane proteins. Creative Proteomics also provides computer virtual screening of protein three-dimensional structures and nuclear magnetic resonance experiments to discover small molecule ligands as potential lead compounds for subsequent functional studies.
Advantages of Nuclear Magnetic Resonance (NMR) Service:
- NMR is the only method that enables research to be performed in solution
- This technology can not only study the structure of proteins, but also the dynamics of proteins
Want to Know about Other Protein Structure Analysis Techniques?
References
- Protein nuclear magnetic resonance techniques. Springer Science & Business Media, 2004.
- Kaplan M, Pinto C, et al. Nuclear magnetic resonance (NMR) applied to membrane–protein complexes. Quarterly reviews of biophysics, 2016, 49.
* For Research Use Only. Not for use in diagnostic procedures.