Protein Crosslinking Services
Online InquiryMost protein interactions in the body are transient. Capturing or freezing these transient interactions allows you to study the proteins involved and how they participate. Protein interactions are an important part of proteomics research. Cross-linking assistants or cross-linking agents can covalently link interacting proteins, thereby freezing those transient and weak interactions, which is conducive to the isolation and characterization of subsequent protein complexes and provides a means for capturing protein-protein complexes.
Creative Proteomics is committed to providing consistent high-quality protein cross-linking services that can be applied to different research projects.
We Provide the Following Services, including but not Limited to:
- Chemical cross-linking mass spectrometry
The protein sample is treated with a chemical cross-linking agent. Two amino acids that are close enough in space to react with the cross-linking agent are linked by covalent bonds, and then the cross-linked products are analyzed using proteomics based on mass spectrometry.
- Amine is crosslinked with amine.
- Crosslinking of thiols with thiols.
- Thiols are crosslinked with amines.
- Thiols / amines are converted to carbohydrates.
- Enzymatic cross-linking services
Enzymes have high substrate specificity. Under physiological conditions, the structural integrity and function of the enzyme are optimal. Therefore, under physiological conditions, enzymes are more favorable in cross-linked proteins.
- Photo-crosslinking services
The photo-crosslinking reaction uses the synthesized photosensitive small molecule compound as a tool probe. Under the irradiation of light of a specific wavelength, a highly active intermediate is produced. It forms a specific irreversible covalent bond with its receptor active site. The photocrosslinking reaction is fast and suitable for in situ reaction. Through the biosynthesis of the organism itself, substrate analogues with photocrosslinking activity can be effectively assimilated into biological macromolecules. It can be used to study the interaction mode and mechanism between biological macromolecules.
Types of Crosslinkers We Can Offer:
- Chemical cross-linking:
- Simple crosslinker: disuccinimidyl suberate (DSS), disuccinimidyl glutarate (DSG), bissulfosuccinimidyl suberate (BS3), bissulfosuccinimidyl glutarate (BS2G)
- Featured Crosslinking Agent:
1) A cross-linking agent having an affinity group. The cross-linking agent is provided with a specific affinity group to be combined with the magnetic beads in the stationary phase. The peptides containing the cross-linking agent can be enriched and the interference of the peptides without the cross-linking agent can be excluded.
2) Crosslinking agent with characteristic ions. In the design of the cross-linking agent, a special group is added, which is easy to fall off under the fragmentation conditions of the mass spectrometer. When the cross-linked peptide is fragmented to generate a secondary spectrum, the group will also fall off, forming a spectral peak.
3) When synthesizing the cross-linking agent, you can choose to add isotope labeling, so as to produce two kinds of light and heavy cross-linking agents. Peak-to-weight isotope labeled peak pairs are formed in the spectrum. This can be used to determine the spectrum containing the cross-linking agent.
- Enzymatic cross-linking
- Transglutaminase
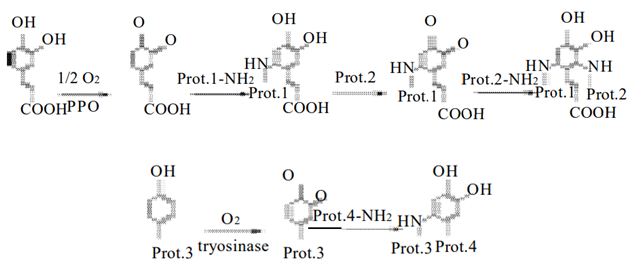
- Polyphenol oxidase (PPO) and peroxidase (POD)
- Lysyl oxidase / amine oxidase
- Photo-crosslinking
- Aromatic azide
- Diazo compounds and diaziridines
- Aromatic diazonium salt compounds
- Benzophenones
Advantages of Protein Crosslinking Services:
- Various cross-linking agents and cross-linking methods to choose from
- Use of cross-linking agents with different arm lengths and different reactive groups can get more information about protein interactions and spatial structure
- Not only high-throughput analysis of multiple protein interactions, but also structural analysis
- Faster than traditional methods and large scale of information output
- The covalent cross-linking action of the cross-linking agent can fix the originally unstable protein interaction. This allows the study of a large class of easily dissociated, loosely structured protein complexes.
- Cross-link mass spectrometry has high sensitivity, low requirements for protein properties, and requires a small absolute amount of sample
- Cross-linked proteins are first digested before being analyzed by mass spectrometry, so they are not affected by the length of the protein itself
Want to Know about Other Subcellular Protein-protein Interaction Analysis Techniques?
References
- Kaake R M, Wang X, et al. A new in vivo cross-linking mass spectrometry platform to define protein–protein interactions in living cells. Molecular & Cellular Proteomics, 2014, 13(12): 3533-3543.
- Ouimet C M, Shao H, et al. Protein Cross-Linking Capillary Electrophoresis for Protein–Protein Interaction Analysis. Analytical chemistry, 2016, 88(16): 8272-8278.
* For Research Use Only. Not for use in diagnostic procedures.