Membrane Protein-Molecule Interaction Analysis
Online InquiryMembrane proteins play a crucial role in communication between the extracellular and intracellular environments through their dynamic interactions. More than half of the cellular proteins are involved in these interactions both spatially and temporally, which determine the fate of the cells that have responded to the diversity of the surrounding environment. Creative Proteomics offers membrane protein-molecular interaction dynamics analysis service based on advanced surface plasmon resonance (SPR) technology. This system combines optical microscopy with molecular interaction techniques and can be used to observe and measure the binding affinity and kinetics of cell membrane proteins with target molecules, opening up a new field of molecular interaction studies.
Surface Plasmon Resonance (SPR) technology
SPR can perform real-time and label-free monitoring of intermolecular interactions, including affinity, kinetics, active concentration, and thermodynamic effects. SPR is a technology that relies on a physical optics phenomenon. A thin layer of biomolecular (dielectric medium) is immobilized on the surface of the sensor chip. The chip surface is then connected to a microchannel system where the flow rate of the sample through the dielectric surface is precisely controlled. The interaction of the molecular ligands with the analytes in a mobile phase solution can lead to a change on the sensor surface which can be detected by SPR phenomena. The detector measures the SPR response of each pixel and maps it to the SPR image. At each pixel, a sensing map is recorded, thus providing more local information. Therefore, the entire process of the sample and ligand interaction is recorded in real-time.
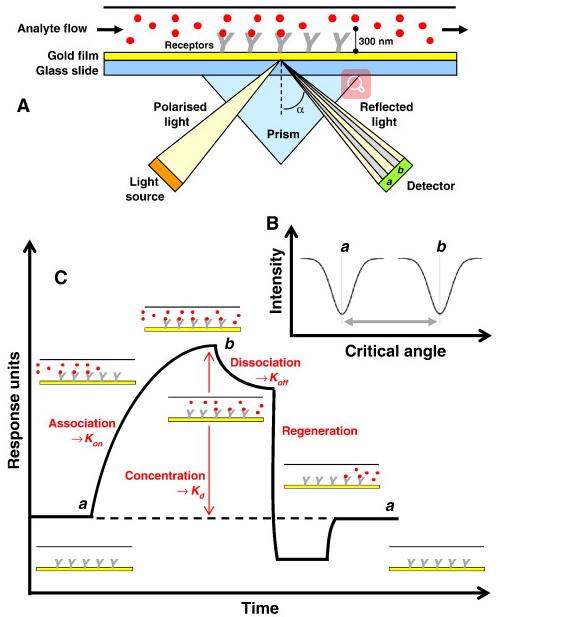 Fig. 1 Schematic illustration of the basic SPR experiment for measuring the binding of an analyte molecule to a receptor molecule. (Patching, Simon G., 2014)
Fig. 1 Schematic illustration of the basic SPR experiment for measuring the binding of an analyte molecule to a receptor molecule. (Patching, Simon G., 2014)
Major advantages of SPR technology
- Label-free analysis
- Low sample requirements
- Stable and repeatable measurements
- In-depth, real-time analysis of the natural dynamics of the sample
- High throughput, high sensitivity, and high selectivity analysis
- Provide accurate outcomes at a low cost
SPR technology in membrane protein-molecule interaction analysis
The SPR system does not require labeling of the observation target and allows for real-time quantitative detection. Cell structure and local binding activity can be visualized simultaneously. The real-time interaction between molecules (such as small molecule drugs) and membrane proteins can be observed and measured in the normal living cell state without extracting cell membrane proteins. This technique makes it possible to study the binding and interaction of cell membrane proteins with target molecules in a sensitive and efficient manner under natural conditions. With our extensive experience and advanced SPR platform, Creative Proteomics provides a comprehensive strategy for investigating the interaction of membrane proteins with a variety of molecules. Our services enable the detection of affinity and kinetics of membrane protein molecules, as well as the determination of affinity for individual cell protein molecules. We are committed to providing our clients with a variety of customized services to meet your special needs.
Application of our service
- Small molecule drug binding screening and analysis with single or multiple cells
- Screening of antibody drugs for binding to single or multiple cells
- Study cell heterogeneity differences
- Other in situ studies at the live cell level
Our clients have direct access to our staff and prompt feedback on their inquiries. If you are interested in our services, please contact us for more details.
Reference
- Patching, Simon G. "Surface plasmon resonance spectroscopy for characterisation of membrane protein-ligand interactions and its potential for drug discovery." Biochimica et Biophysica Acta (BBA)-Biomembranes 1838.1 (2014): 43-55.
* For Research Use Only. Not for use in diagnostic procedures.