Bimolecular Fluorescence Complementation Service
Online InquiryBimolecular Fluorescence Complementation (BiFC) technology divides the fluorescent reporter protein into two sections without fluorescence according to rules. Fusion with bait protein and capture protein, respectively. Only when the bait protein and the capture protein interact, the two incomplete fluorescent reporter proteins will form a complete reporter protein and emit fluorescence. This technology can intuitively and quickly determine the localization and interaction of the target protein in living cells, and is suitable for a variety of tissues and cells such as prokaryotes, fungi, plants, and animals.
Creative Proteomics has extensive proteomics experience and can provide bi-molecular fluorescence Complementation technology for pharmaceutical companies or research institutions. This method can not only verify the existence of protein-protein interactions, but also study the location of intracellular protein interactions.
The Principle and Process of Bimolecular Fluorescence Complementation Technology
The fluorescent protein is cleaved at the appropriate site to form two non-fluorescent polypeptides (VN and VC). When these two fragments are co-expressed intracellularly or mixed in vitro, they cannot spontaneously assemble into a complete fluorescent protein. When the excitation light of the fluorescent protein is excited, no fluorescence can be generated. The two peptides are fused to bait protein and capture protein, respectively. If the bait protein and the capture protein can interact, two incomplete fragments of the fluorescent reporter protein will be close to each other, regenerating the active fluorescent protein. When the excitation light of the fluorescent protein is excited, it can generate fluorescence. Finally, observe the presence of fluorescence through a fluorescence microscope to determine whether there is interaction between the bait protein and the capture protein.
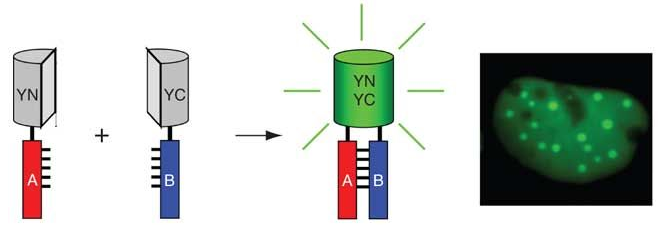 Figure 1 The Principle of Bimolecular Fluorescence Complementation (Kerppola et al, 2006)
Figure 1 The Principle of Bimolecular Fluorescence Complementation (Kerppola et al, 2006)
Characteristics of Bimolecular Fluorescence Complementation Protein Interaction Service:
- The protein interaction can be directly observed under the microscope and does not depend on other secondary effects.
- This interaction can be observed in living cells, excluding false positive results due to cell lysis or fixation.
- The protein is expressed in an environment close to physiological conditions, and the expression level and characteristics such as post-translational modifications are very close to endogenous proteins.
- No special theoretical ratio of proteins is required, and interactions between different subgroup proteins can be detected
- Multicolor BiFC technology can not only detect the formation of multiple protein complexes at the same time, but also compare the strength of interactions between different proteins.
- The BiFC system is temperature sensitive. When the temperature is high, it is difficult for the fragments to complement each other to form a complete fluorescent protein. Complementation effects are generally good at temperatures below 30 °C. The lower the temperature, the better the complementarity between the fragments. This brings unfavorable factors to study the protein-protein interaction of cells under physiological conditions. Currently, only three BiFC systems based on venus, citrine and cerulean can achieve fragment complementation at physiological temperature conditions.
- Failure to observe interactions in real time
Want to Know about Other Protein-protein Interaction Analysis Techniques?
References
- Goto-Yamada S, Hikino K, Nishimura M, et al. Bimolecular Fluorescence Complementation with Improved Gateway-Compatible Vectors to Visualize Protein–Protein Interactions in Plant Cells//Two-Hybrid Systems. Humana Press, New York, NY, 2018: 245-258.
- Zhou F J. Using Bimolecular Fluorescence Complementation to Visualize Protein-Protein Interactions of Potassium Channels in Live Cells. Brandeis University, 2016.
- Kerppola T K. Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nature protocols, 2006, 1(3): 1278-1286.
* For Research Use Only. Not for use in diagnostic procedures.