Mitochondrial Protein Acetylation Analysis
Online InquiryProtein acetylation in mitochondria has come into focus, with studies showing that 63% of mitochondrially localized proteins contain lysine acetylation sites. Recent studies have also suggested that dysfunctional protein acetylation in mitochondria may contribute to the development of various diseases, such as neurodegenerative disorders, cardiovascular disease, cancer, obesity, and diabetes. Based on subcellular prefractionation methods, efficient acetyl peptide enrichment, and advanced quantitative mass spectrometry (MS) techniques, Creative Proteomics can help our global customers to perform deep and accurate mitochondrial protein acetylation analysis.
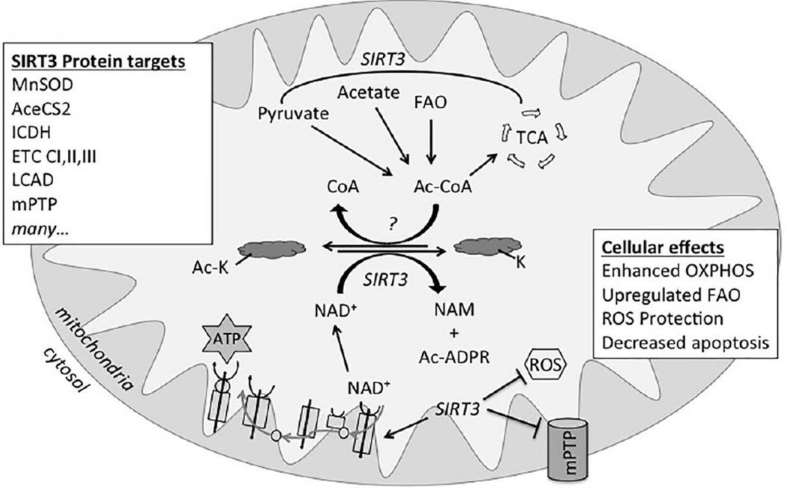 Fig. 1 Reversible lysine acetylation. (Stram, Amanda R., and R. Mark Payne. 2016)
Fig. 1 Reversible lysine acetylation. (Stram, Amanda R., and R. Mark Payne. 2016)
Mitochondrial acetylation-related diseases
Mitochondria, as the organelles of cellular energy control, are involved in the catabolism and anabolism of glucose, fatty acids and amino acids, regulating cellular physiological and pathological activities, and of course, mitochondrial acetylation is involved in the development of many diseases. Acetylation of mitochondrial proteins can occur by both enzymatic and non-enzymatic pathways, with non-enzymatic mechanisms being the major ones. Hyperacetylation of mitochondrial proteins accelerates glycolytic reactions, which in turn promotes malignant tumorigenesis. In addition, tissues and organs with high metabolic rates are more sensitive to mitochondrial dysfunction, and mitochondrial hyperacetylation has been shown to be involved in the development of aging, neurodegenerative diseases, liver disease, kidney disease, heart disease and other metabolic diseases. The deacetylation function of SIRT, which inhibits mitochondrial hyperacetylation, has led to the current use of SIRT3 as a promising therapeutic target for a variety of human diseases as well.
Mitochondrial protein acetylation analysis at Creative Proteomics
Lysine acetylation has emerged as a key post-translational modification (PTM) for regulating mitochondrial metabolism. As a reliable partner for pharmaceutical companies, research institutions, and government agencies, Creative Proteomics can provide you with a wide range of mitochondrial protein modification analysis services, including mitochondrial protein acetylation analysis. Our service is based on the combination of acetylation-specific antibody enrichment technology and high-precision liquid chromatography-tandem mass spectrometry (LC-MS/MS), allowing large-scale identification and quantitative analysis of acetylated proteins and acetylation sites. Our mitochondrial protein acetylation analysis service has proven to be effective, reliable, and reproducible. Upon receipt of your sample, we will manage all aspects of the project, providing analysis and reports until the customer is satisfied.
Quantitative acetylproteome analysis
- Experimental consultation and design
- Sample preparation and isolation of ultra-pure mitochondria
- Mitochondrial acetylproteome sample preparation (TMT or iTRAQ).
- LC-MS/MS analysis
- Raw data analysis
- Bioinformatics analysis
Sample requirements
- Acceptable samples:
-Cell samples (cell volume > 107)
-Tissue samples (animal tissue >2000 mg, plant tissue >1000 mg)
-Body fluid samples (blood, urine)
-Microorganisms dry weight > 200 mg
- Sample shipping:
Sufficient amount of dry ice for shipping, or consult our technical staff before sending samples
- We will always test the samples provided by you before the formal experiment
Main benefits of our service
- Highly stable method for protein extraction
- High-performance mass spectrometry system
- Multiple relevant high-quality protein databases
- Comprehensive data analysis, including statistical analysis, functional annotation, enrichment analysis, clustering analysis, and network analysis
References
- Parodi-Rullán, Rebecca M., Xavier R. Chapa-Dubocq, and Sabzali Javadov. "Acetylation of mitochondrial proteins in the heart: the role of SIRT3." Frontiers in Physiology 9 (2018): 1094.
- Stram, Amanda R., and R. Mark Payne. "Post-translational modifications in mitochondria: protein signaling in the powerhouse." Cellular and molecular life sciences 73 (2016): 4063-4073.
* For Research Use Only. Not for use in diagnostic procedures.