Mitochondrial Functional Analysis Methods
Online InquiryMitochondria are semi-autonomous organelles surrounded by a double layer of highly specialized unit membranes in eukaryotic cells that produce energy to maintain normal cellular physiological activities. They are involved in the regulation or signaling of growth, development, metabolism, aging, disease, death, and biological evolution, and are also involved in intracellular Ca2+ homeostasis, reactive oxygen species production, and cytochrome C release.
Mitochondrial function can be directly impaired by hypoxia and many of the mediators of the associated systemic inflammatory response. Mitochondrial dysfunction is also associated with endoplasmic reticulum (ER) stress due to accumulation of misfolded or unfolded proteins caused by reduced ER-associated protein degradation (ERAD). Misfolded or unfolded proteins are translocated from the ER lumen to the cytoplasm. Here they are ubiquitinated and then eliminated, triggering endoplasmic reticulum stress and leading to mitochondrial fragmentation and mitochondrial dysfunction. When mitochondria are damaged, the mitochondrial membrane potential is lost and the mitochondrial permeability transition pore (MPTP) opens. Under the action of different pathogenic stimuli, mitochondria are highly susceptible to structural and functional damage, which directly affects the normal function of other cellular tissues of the body.
There are several methods for testing mitochondrial function.
Mitochondrial Membrane Potential Assay
The mitochondrial membrane potential assay is a technique used to measure the voltage difference across the inner mitochondrial membrane, which is an important indicator of mitochondrial function and health. This measurement is typically performed using a dye that is sensitive to changes in membrane potential, and fluorescence spectrophotometry is used to quantify the change in fluorescence of the dye. The mitochondrial membrane potential assay provides important information about the energy status of cells and can be used to study various physiological processes, including cell metabolism, apoptosis, and oxidative stress.
MPTP Detection
The MPTP assay is a technique used to measure the opening of the MPTP, a high-conductance channel in the inner mitochondrial membrane. The opening of the MPTP leads to the release of pro-apoptotic factors, and is a hallmark of apoptosis and cellular injury.
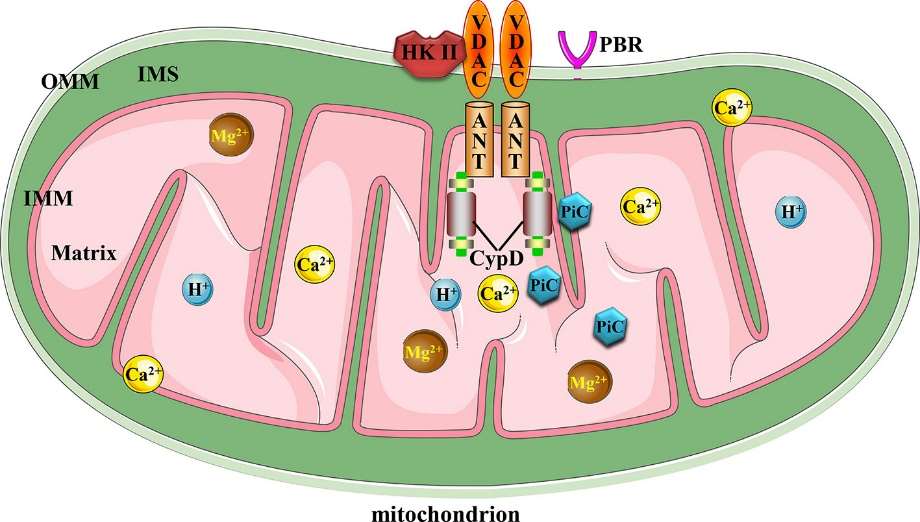 Canonical mitochondrial
MPTP molecular structure (Li et al., 2020).
Canonical mitochondrial
MPTP molecular structure (Li et al., 2020).
The mitochondrial membrane potential (MPTP) can be detected using different methods, including:
- Spectrophotometric methods: These methods use fluorescent dyes, such as tetramethylrhodamine ethyl ester (TMRE) or JC-1, that are sensitive to changes in membrane potential and emit fluorescence that can be quantified using spectrophotometry.
- Active substance labeling methods: These methods use radioisotopes or stable isotopes to label active substances, such as ATP, that are synthesized by the mitochondria. The amount of labeled substance produced can be used to calculate the mitochondrial membrane potential.
- Membrane patch clamp methods: These methods use patch clamp electrophysiology techniques to measure the changes in membrane potential directly. The membrane potential is manipulated using electrical stimuli, and the response is recorded using a patch clamp amplifier.
Each method has its own advantages and limitations, and the choice of method will depend on the research question, the type of cells being studied, and the available equipment and expertise.
Mitochondrial Ca2+ Measurement
Methods for the detection of mitochondrial Ca2+ include precipitation, electrochemical electrochemical analysis, EDTA chelation titration, flame photometry, atomic absorption spectrophotometry, etc.
- Precipitation: This method involves the use of a calcium-specific reagent, such as calcium oxalate, to precipitate calcium ions, which can then be quantified by spectrophotometry or other analytical methods.
- Electrochemical analysis: This method involves the use of a microelectrode or other electrical sensor to measure changes in the concentration of calcium ions in the mitochondrial matrix.
- EDTA chelation titration: This method involves the use of ethylenediaminetetraacetic acid (EDTA), a chelating agent that binds to calcium ions, to determine the total calcium content in mitochondria.
- Flame photometry: This method uses a flame to excite the atoms of a sample and measure the intensity of light emitted, which can be used to quantify the concentration of calcium ions in mitochondria.
- Atomic absorption spectrophotometry: This method uses the absorption of light by atoms to determine the concentration of specific elements, such as calcium, in a sample.
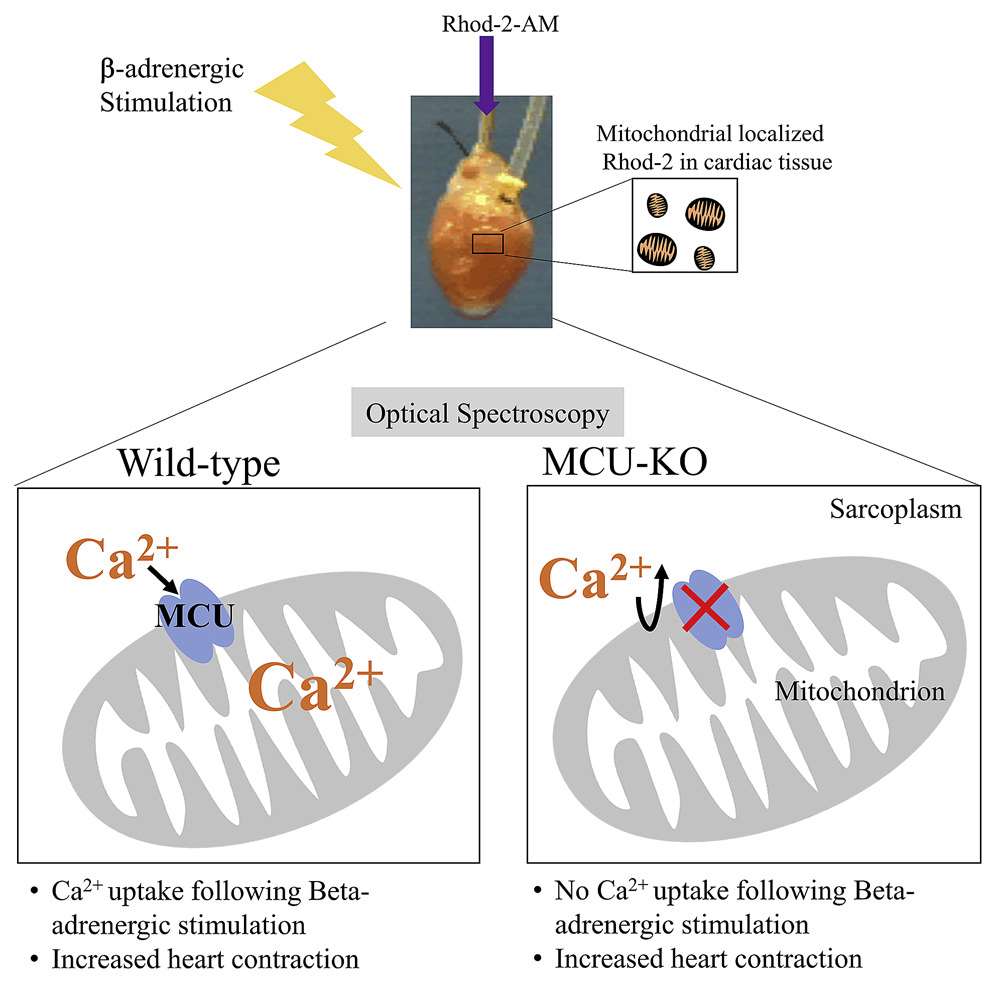 Optical Spectroscopy (Kosmach et al., 2021)
Optical Spectroscopy (Kosmach et al., 2021)
Mitochondrial ATP Assay
Mitochondrial ATP assay is used to measure the amount of ATP (adenosine triphosphate) produced by mitochondria. This measurement provides insights into cellular energy metabolism and mitochondrial function.
Enzymatic methods for mitochondrial ATP assay use ATP-dependent luciferase or luciferin to generate light, which is proportional to the amount of ATP present. Advantages of this method include high sensitivity, ease of use, and the ability to measure ATP in real-time. However, this method is limited by the interference from other bioluminescent compounds and the requirement for specialized equipment.
High-performance liquid chromatography (HPLC) is another method for measuring mitochondrial ATP. In this method, ATP is separated from other cellular components using liquid chromatography and then quantified using UV/Vis spectrophotometry. Advantages of HPLC include high precision and accuracy, and the ability to measure ATP in the presence of other cellular components. However, this method is more time-consuming and requires specialized equipment compared to enzymatic methods.
Mitochondrial Respiratory Chain Complex Activity Assay
Understanding the activity of the respiratory chain complex is essential for the study of cellular energy production processes and the role of mitochondria in various physiological and pathological processes. Defects in the activity of specific respiratory chain complexes have been associated with various mitochondrial diseases. Measuring the activity of the complexes can help diagnose these diseases and monitor disease progression. The activity of respiratory chain complexes can be altered by various factors, such as drugs, toxicants and other interventions. Measuring the activity of complexes can help assess the effectiveness and toxicity of these interventions.
The activity of mitochondrial respiratory chain complexes can be determined through various methods, including:
- Enzyme assays - measuring the rate of oxygen consumption, or the rate of ATP production, using the complexes and substrates as enzymes.
- Spectrophotometry - monitoring changes in absorbance of specific dyes that are sensitive to changes in redox state.
- Fluorometry - monitoring changes in fluorescence intensity of specific dyes, such as JC-1, which are sensitive to changes in membrane potential.
- Blue native gel electrophoresis - separating respiratory chain complexes based on their molecular weight, followed by detection of specific complexes through immunoblotting.
- Isolated mitochondria respirometry - measuring oxygen consumption of isolated mitochondria in the presence of various respiratory chain substrates.
- Cytochrome c oxidase activity assays - measuring the rate of cytochrome c oxidase activity, which is a specific marker for complex IV activity.
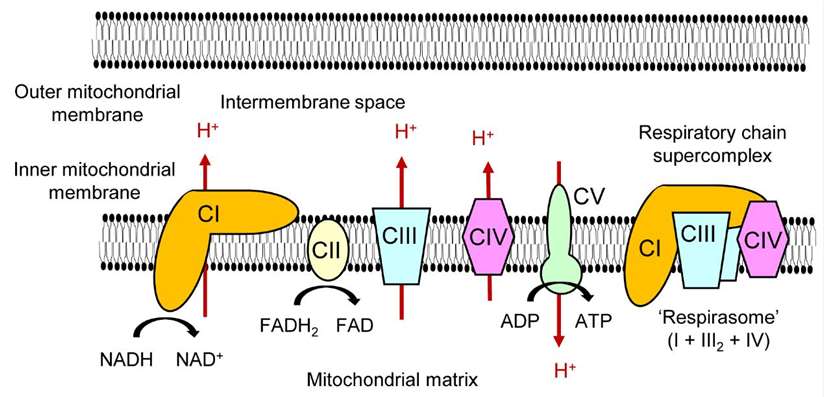 Mitochondrial OXPHOS complexes and a respiratory chain supercomplex (Kobayashi et al.,
2020).
Mitochondrial OXPHOS complexes and a respiratory chain supercomplex (Kobayashi et al.,
2020).
Reactive Oxygen Species Assay
Reactive oxygen species (ROS) are highly reactive molecules that can cause cellular damage. Measuring ROS levels can provide important information about cellular oxidative stress and oxidative damage. For example, high levels of ROS can cause oxidative stress, which can cause cell damage and contribute to various diseases such as cancer, cardiovascular disease, and neurodegenerative diseases. Measuring ROS levels can provide information on cellular oxidative stress levels. ROS levels can vary based on a variety of factors, including drugs, poisons, and other interventions, and measuring ROS levels can help assess the efficacy and toxicity of these interventions.
Methods for measuring reactive oxygen species include:
- Chemiluminescence: This method measures the light emitted during the reaction between ROS and a luminol-like substrate.
- Fluorometry: This method measures changes in fluorescence intensity of specific dyes that are sensitive to oxidative stress, such as 2',7'-dichlorofluorescein diacetate (DCFH-DA).
- Electron spin resonance (ESR) spectroscopy: This method measures the changes in the electron spin of specific radicals, such as the hydroxyl radical, that are generated by ROS.
- HPLC-MS methods: These methods measure the levels of specific ROS-generated compounds.
Each method has its own advantages and disadvantages. Chemiluminescence and fluorometry are easy to perform and can provide rapid measurements of ROS levels. ESR spectroscopy can provide detailed information about specific radical species, but is more complex and time-consuming. The high-performance liquid chromatography (HPLC) based method allows highly sensitive and specific detection of ROS species, allowing quantification of low levels of ROS in biological samples. The ability to measure multiple ROS species simultaneously in a single analysis provides a comprehensive picture of the cellular redox state. The HPLC-MS method has a wide linear dynamic range, allowing the detection and quantification of ROS species over a wide range of concentrations.
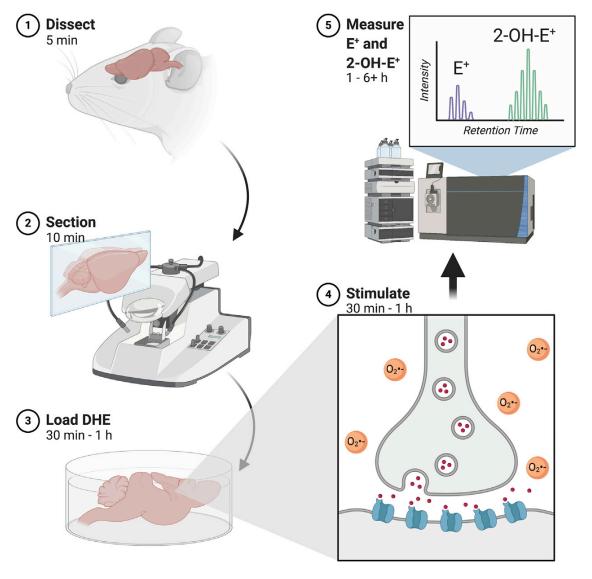 Evaluating redox homeostasis involves gauging the levels of reactive oxygen
species (ROS) and reactive nitrogen species (RNS) directly in tissues and cells (Vasavda et al., 2021).
Evaluating redox homeostasis involves gauging the levels of reactive oxygen
species (ROS) and reactive nitrogen species (RNS) directly in tissues and cells (Vasavda et al., 2021).
References
- Li, Yangxin, et al. "Mitochondrial MPTP: a novel target of ethnomedicine for stroke treatment by apoptosis inhibition." Frontiers in pharmacology 11 (2020): 352.
- Kosmach, Anna, et al. "Monitoring mitochondrial calcium and metabolism in the beating MCU-KO heart." Cell Reports 37.3 (2021): 109846.
- Kobayashi, Ami, et al. "Mechanisms underlying the regulation of mitochondrial respiratory chain complexes by nuclear steroid receptors." International journal of molecular sciences 21.18 (2020): 6683.
- Vasavda, Chirag, Solomon H. Snyder, and Bindu D. Paul. "Quantitative measurement of reactive oxygen species in ex vivo mouse brain slices." STAR protocols 2.1 (2021): 100332.
Related Services
* For Research Use Only. Not for use in diagnostic procedures.