Mitochondria and Health
Online InquiryMitochondria: Orchestrators of Cellular Energetics
Adenosine triphosphate (ATP), the cell's main energy source, is produced by the active organelles known as mitochondria. They are unrivaled in their capacity to use oxidative phosphorylation to transform nutrients into ATP, ensuring the steady flow of energy needed for cellular functions. An extensive electron transport chain (ETC) found in mitochondria uses electron energy to fuel ATP synthesis.
Oxidative Stress: A Double-Edged Sword
Oxidative stress develops when there is an imbalance between the creation of reactive oxygen species (ROS) and the body's antioxidant defense mechanisms. ROS are a byproduct of cellular metabolism and are a natural consequence. Although ROS play an important role as signaling molecules in physiological processes, an excessive amount of them can cause cellular harm and dysfunction.
Analyzing the Relationship Between Mitochondria and Oxidative Stress
Oxidative Phosphorylation and ROS Generation
Numbers of electron transport chain (ETC) complexes are involved in the process of oxidative phosphorylation, which takes place inside the inner mitochondrial membrane. As electrons move along the ETC during this process, a proton gradient is eventually created across the inner mitochondrial membrane. The creation of ATP, the cell's main source of energy, is fueled by this gradient.
However, the efficiency of the electron transport through the ETC is not always flawless. It's possible for some electrons to prematurely leave the chain, especially at Complex I and III. These leaking electrons can cause molecular oxygen to react, producing reactive oxygen species (ROS) such superoxide anion (O2) and hydrogen peroxide (H2O2). Low amounts of ROS act as critical signaling molecules in cellular processes like apoptosis, immunological response, and stress adaptation under normal conditions.
The Role of Antioxidant Systems
Cells have a variety of antioxidant defense mechanisms to balance the possible negative effects of ROS. These include enzyme-based antioxidants like catalase, glutathione peroxidase, and superoxide dismutase (SOD), as well as non-enzyme-based antioxidants like glutathione and vitamins C and E. Together, these systems detoxify and neutralize ROS to stop excessive oxidative damage to cellular components.
Oxidative Stress and Mitochondrial Dysfunction
While cellular antioxidant systems can control low levels of ROS, excessive ROS production can overwhelm these defenses and result in oxidative stress. When the equilibrium between ROS generation and antioxidant capacity is upset, oxidative stress results, which causes oxidative damage to lipids, proteins, and DNA. Due to their closeness to the ROS-generating sites within the ETC, mitochondria are particularly susceptible to oxidative damage.
Effects of Oxidative Stress on Cells and Health
Oxidative stress has various of negative impacts on cellular constituents and general health because it results from an imbalance between the generation of reactive oxygen species (ROS) and the cell's capacity to neutralize them. This occurrence, also known as oxidative damage, has been linked to a variety of cellular dysfunctions and is a contributing factor in a wide range of medical diseases. Developing solutions to lessen oxidative stress's harmful effects requires an understanding of the complex effects it has on cells and health.
Implications for Cellular Health
The complex balancing act between oxidative stress and mitochondrial function has significant effects on cellular health and general wellbeing. Chronic oxidative stress has been linked to various of clinical conditions.
In addition, oxidative stress plays a role in the aging process itself. Over time, the slow buildup of oxidative damage can cause cellular malfunction and senescence, which ultimately accelerates tissue aging and the development of age-related illnesses.
DNA Damage and Mutation
Because oxidative stress directly oxidizes DNA bases, genomic stability is seriously threatened. This can impair the precision of DNA replication and repair by causing DNA strand breakage, cross-links, and alterations.
Cellular Senescence
Oxidative stress quickens cellular senescence, an irreversible growth halt state. Senescence is caused by signaling pathways that are triggered by the buildup of oxidative damage over time. Senescent cells stop dividing and functioning normally, which accelerates tissue aging and age-related diseases.
Mitochondrial Dysfunction
Because they are both generators and targets of oxidative stress, mitochondria are especially susceptible to its effects. Proteins, lipids, and DNA that have been oxidatively damaged might lose their functionality and limit the production of energy. Because poor energy production causes more ROS to be produced, this mitochondrial malfunction can set off a vicious loop that worsens oxidative stress.
Inflammation and Immune Response
Oxidative stress activates inflammatory pathways, setting the stage for chronic low-grade inflammation. Creative Proteomics' studies have elucidated how ROS-triggered signaling cascades lead to the secretion of pro-inflammatory cytokines. This sustained inflammation, known as inflammaging, contributes to a wide spectrum of health issues, including cardiovascular diseases, neurodegenerative disorders, and metabolic syndrome.
Protein and Lipid Damage
Proteins and lipids, essential components of cellular structure and function, are susceptible to oxidative modification. Creative Proteomics' analyses have revealed that oxidative stress can lead to protein misfolding, aggregation, and dysfunction. Lipid peroxidation, driven by ROS, disrupts the integrity of cellular membranes, compromising their fluidity and selective permeability. Such damage can impair cellular signaling, transport, and overall cell viability.
Cancer Development
Oxidative stress has been implicated in the initiation and progression of cancer. As DNA damage accumulates due to oxidative stress-induced mutations, cells may acquire oncogenic alterations that promote uncontrolled growth and tumorigenesis.
Neurodegenerative Disorders
The brain's high metabolic rate and abundance of lipid-rich membranes make it particularly susceptible to oxidative stress. Creative Proteomics' investigations highlight how oxidative damage contributes to the pathogenesis of neurodegenerative diseases, such as Alzheimer's and Parkinson's. Oxidative stress disrupts neuronal function, promotes protein aggregation, and triggers neuroinflammation, ultimately contributing to cognitive decline and motor dysfunction.
Inflammation and Disease Risks Triggered by Excessive Mitochondrial Production
The intricate relationship between mitochondrial function and cellular health extends beyond energy production, encompassing crucial aspects of inflammation and disease susceptibility. Understanding the intricate mechanisms that underlie this phenomenon is paramount for devising strategies to mitigate its impact on human health.
Mitochondrial Dysfunction and Inflammatory Signaling
Excessive mitochondrial production, often driven by increased cellular demands or stressors, can lead to mitochondrial dysfunction and subsequent release of damage-associated molecular patterns (DAMPs). These DAMPs act as danger signals that activate the innate immune system and trigger pro-inflammatory responses.
Inflammasome Activation
One of the critical consequences of excessive mitochondrial production is the activation of inflammasomes, multi-protein complexes responsible for processing and releasing pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and IL-18. Mitochondrial dysfunction can lead to the release of mitochondrial ROS, mitochondrial cardiolipin, and mtDNA, all of which can activate different types of inflammasomes. This activation fuels the production of potent pro-inflammatory cytokines, further exacerbating inflammation and potentially leading to tissue damage.
Chronic Inflammation and Disease Risks
The persistent activation of inflammatory pathways due to excessive mitochondrial production can contribute to chronic low-grade inflammation, a hallmark of various diseases. As demonstrated by Creative Proteomics' research, this chronic inflammation, often referred to as inflammaging, plays a pivotal role in the development and progression of numerous health conditions, including as follows.
- Cardiovascular Diseases. Excessive mitochondrial ROS production and subsequent inflammation promote endothelial dysfunction and vascular inflammation. This environment contributes to atherosclerosis, hypertension, and other cardiovascular disorders.
- Metabolic Syndrome. Oxidative stress triggered by excessive mitochondrial production has been linked to insulin resistance, adipose tissue inflammation, and obesity-related metabolic disturbances.
- Neurodegenerative Diseases. Chronic inflammation resulting from mitochondrial dysfunction may contribute to neurodegenerative diseases like Alzheimer's and Parkinson's.
- Mitochondrial-Nuclear Crosstalk and Inflammation. Mitochondrial dysfunction and excessive production can trigger retrograde signaling pathways that communicate with the nucleus. This nuclear-mitochondrial crosstalk further amplifies the inflammatory response, contributing to a self-perpetuating cycle of inflammation and mitochondrial dysfunction.
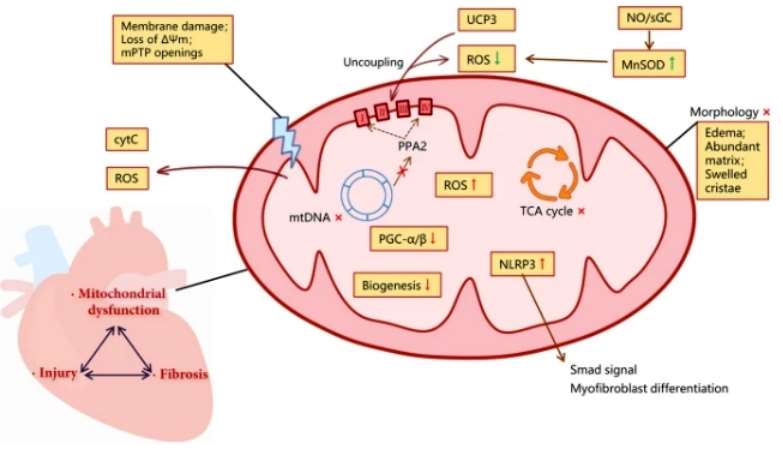 Mitochondrial dysfunction in cardiac fibrosis (Li X,et al,.2020)
Mitochondrial dysfunction in cardiac fibrosis (Li X,et al,.2020)
Therapeutic Implications
Targeting mitochondrial dysfunction and excessive production could serve as a strategy to modulate inflammation and mitigate disease risks. Developing interventions that restore mitochondrial health, enhance antioxidant defenses, or attenuate inflammasome activation holds promise for alleviating the burden of chronic inflammation-related diseases.
Creative Proteomics is dedicated to mitochondrial research and analysis, and provides comprehensive mitochondrial-related research services such as mitochondrial proteomics, mitochondrial lipidomics, mitochondrial metabolomics, mitochondrial phenotyping, mitochondrial functional analysis, and so on. We play an important role in helping researchers to promote mitochondria-related research.
Reference
- Li X, Zhang W, Cao Q, Wang Z, Zhao M, Xu L, Zhuang Q. Mitochondrial dysfunction in fibrotic diseases. Cell Death Discov. 2020 ;6:80.
Related Services
* For Research Use Only. Not for use in diagnostic procedures.