Exosome Extraction and Purification Methods
Online InquiryDifferential centrifugation
Differential centrifugation is one of the most common techniques for exosome isolation. The method involves several steps, including 1) low-speed centrifugation to remove cells and apoptotic debris, 2) higher-speed centrifugation to eliminate larger vesicles, and finally, 3) high-speed centrifugation to precipitate exosomes.
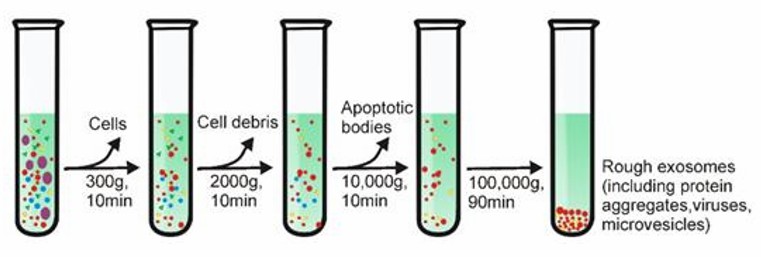 Differential Centrifugation (Yang et al., 2020)
Differential Centrifugation (Yang et al., 2020)
There is a significant correlation between the viscosity of the sample and the purity of the isolated exosomes. Therefore, biological samples with high viscosity (e.g. plasma and serum samples) require longer ultracentrifugation steps and higher centrifugation speeds.
Density gradient centrifugation
This method combines ultracentrifugation with sucrose density gradients to achieve separation of exosomes from non-vesicular particles, such as proteins and protein/RNA aggregates. This method separates vesicles from particles of different densities and enables the extraction of exosomes with low content. However, the appropriate centrifugation time is very important and contaminating particles can still be found in exosomes if they have similar densities.
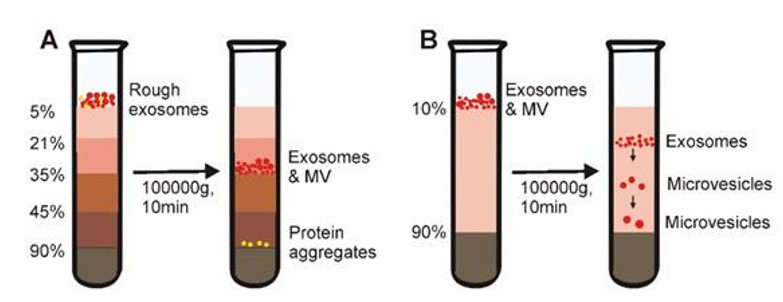 Density gradient centrifugation (Yang et al., 2020)
Density gradient centrifugation (Yang et al., 2020)
Size-exclusion chromatography (SEC)
SEC is based on size rather than molecular weight to achieve sample separation. A column filled with porous polymer microspheres is applied. When a liquid sample is passed through a column filled with a porous multi-channel gel, substances of small radius in the sample can enter the channels with longer paths, and substances of larger radius that cannot enter the channels move directly around the gel. Particulate matter of different sizes is retained in the gel for different times, resulting in the separation of substances. Size exclusion chromatography allows precise separation of small and large molecules.
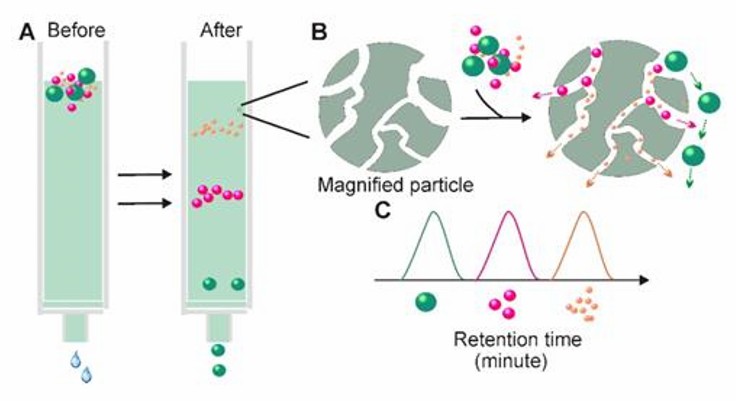 Size-exclusion chromatography (Yang et al., 2020)
Size-exclusion chromatography (Yang et al., 2020)
In addition, different elution solutions can be applied to the method. Chromatographic separation has been shown to offer additional advantages over centrifugal methods, as exosomes separated by chromatography are not affected by shear forces, which may alter the structure of vesicles. Currently, SEC is a widely accepted technique for the separation of exosomes from blood and urine. However, the method is time-consuming and not suitable for processing large numbers of samples.
Polymer precipitation method
Highly hydrophilic polymers (polyethylene glycol PEG is more commonly used) are used to competitively bind water molecules, resulting in no room for exosomes, reduced solubility, and subsequent precipitation. The highly hydrophilic polymer is added to samples with larger debris particles such as cell debris and apoptotic vesicles removed, incubated overnight at 4°C, and then exosomes are collected by low-speed centrifugation. Precipitation with this polymer has many advantages, including low impact on the isolated exosomes and pH neutrality.
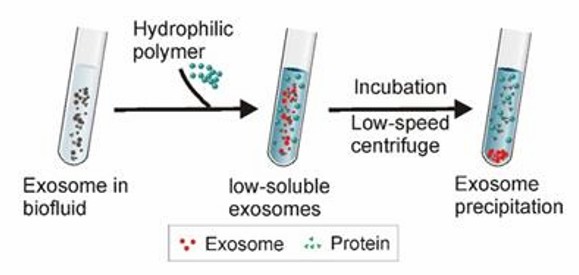 Polymer precipitation (Yang et al., 2020)
Polymer precipitation (Yang et al., 2020)
Immunomagnetic bead method
Antibodies used to identify exosome markers are immobilized on the surface of magnetic beads, and then samples containing exosomes are incubated with the beads. The exosomes bind to the magnetic beads to form a magnetic bead-exosome complex, and then the exosomes are finally eluted and collected. This method is not suitable for obtaining exosomes from a large number of samples.
Ultrafiltration technology
Ultrafiltration allows separation by applying external pressure to create a pressure difference between the two sides of the ultrafiltration membrane (which is upgraded to an ultrafine nanofilm).
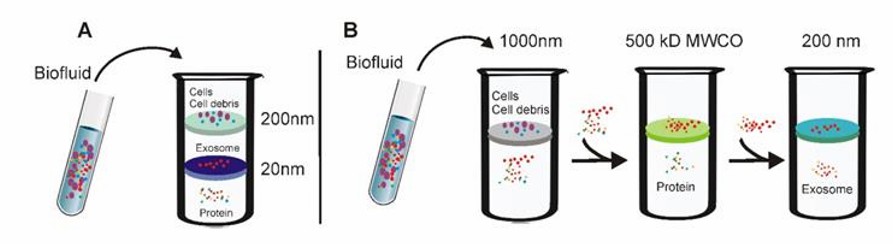 Tandem ultrafiltration and Sequential ultrafiltration (Yang et al., 2020)
Tandem ultrafiltration and Sequential ultrafiltration (Yang et al., 2020)
- Tandem ultrafiltration
Larger cells as well as cell debris are retained using ultrafiltration membranes with 200 nm pore size. Proteins are filtered using ultrafiltration membranes with a pore size of 20 nm, while vesicles of 20-200 nm are retained on the ultrafiltration membrane.
- Sequential ultrafiltration
Cells and cell debris are retained using ultrafiltration membranes with a pore size of 1000 nm. Filtration of small particles, such as proteins, using ultrafiltration membranes with 5000 Da MWCO. Particulate material retained by 5000 Da MWCO ultrafiltration membranes is passed through 200 nm pore size ultrafiltration membranes, allowing exosomes to be filtered and collected.
The ultrafiltration technique is simple and efficient, with low instrumentation requirements and the ability to extract particles of a specific size by adjusting the pore size of the ultrafiltration membrane. However, there are still problems. Firstly, externally applied pressure can lead to impairment of exosome morphology and function. Secondly, clogging and retention of vesicles on the membrane can shorten the life of expensive ultrafiltration membranes and significantly reduce the purity and yield of exosomes.
- Tangential flow filtration
The sample is fed parallel to the membrane surface. A certain pressure perpendicular to the membrane surface is also applied so that substances smaller than the membrane pore size can pass through the membrane, while substances larger than the pore size are left parallel, effectively minimizing the possibility of clogging.
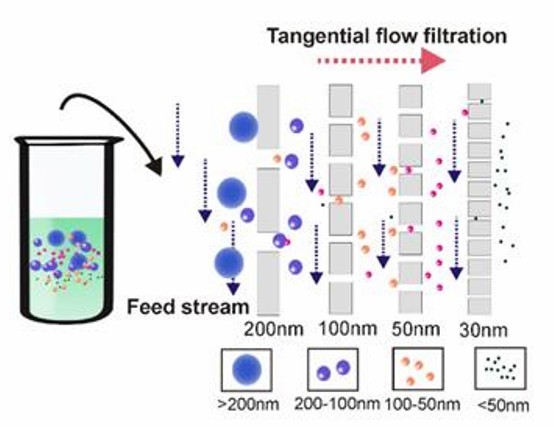 Tangential flow filtration technology (Yang et al., 2020)
Tangential flow filtration technology (Yang et al., 2020)
Microfluidics
Microfluidic technology has been successfully applied to the biological field in recent years, featuring small sample volume requirements, high sensitivity, rapid detection, high throughput, and low cost of detection. It can be combined with traditional exosome extraction techniques as well as with acoustic waves and microfluidic viscoelasticity to provide new solutions for exosome extraction.
Acoustofluidics, a combination of microfluidics and acoustics, can effectively sort and manipulate particles such as cells. The viscous force is proportional to the radius of the particle, while the acoustic radiation force is proportional to the volume of the particle. Under the combined effect of acoustic radiation and viscous force, particles of different sizes will move to different exits, thus achieving particle separation.
The acoustic fluid-based exosome separation method has good biological properties of the obtained exosomes, good purity (~98%) and recovery (~82%), and requires less sample volume.
Creative Proteomics provides exosome analysis related services, including
- Exosome isolation and purification
- Exosome identification
- Exosome proteomics analysis
- Exosome metabolomics analysis
- Exosome lipidomic analysis
Contact us to learn more.
Reference
- Yang, D., Zhang, W., et al. (2020). Progress, opportunity, and perspective on exosome isolation-efforts for efficient exosome-based theranostics. Theranostics, 10(8), 3684.
* For Research Use Only. Not for use in diagnostic procedures.