Mitochondrial Protein Sample Extraction Method
Online InquiryMitochondria are an important organelle found in eukaryotes. They are the center of cellular energy metabolism and also play a signaling role in many life activities, including apoptosis and autophagy, regulation of calcium homeostasis, and reactive oxygen species signaling. Defective mitochondrial function and signaling disorders have been shown to be associated with many major diseases, including cancer, neurodegenerative diseases, metabolic disorders, and cardiomyopathies.
Mitochondrial core protein fractions have been constructed and dysfunction of these proteins has been linked to over 150 different diseases. However, there are still hundreds of mitochondrial proteins whose characteristics are either lacking in understanding or completely unknown. The molecular mechanisms of about 40% of mitochondria-related diseases are unknown, severely hindering the diagnosis and drug development of these diseases and leaving the patients concerned without drugs for a long time. Mass spectrometry-based analysis of mitochondrial proteomics will facilitate progress in the study of mitochondrial protein functional mechanisms.
As a semi-autonomous organelle in eukaryotic cells, the bilayer membrane structure of mitochondria also adds a certain degree of difficulty to researchers. The preparation of mitochondrial protein samples is very different from other cellular proteins. Two methods of mitochondrial protein sample preparation are described below.
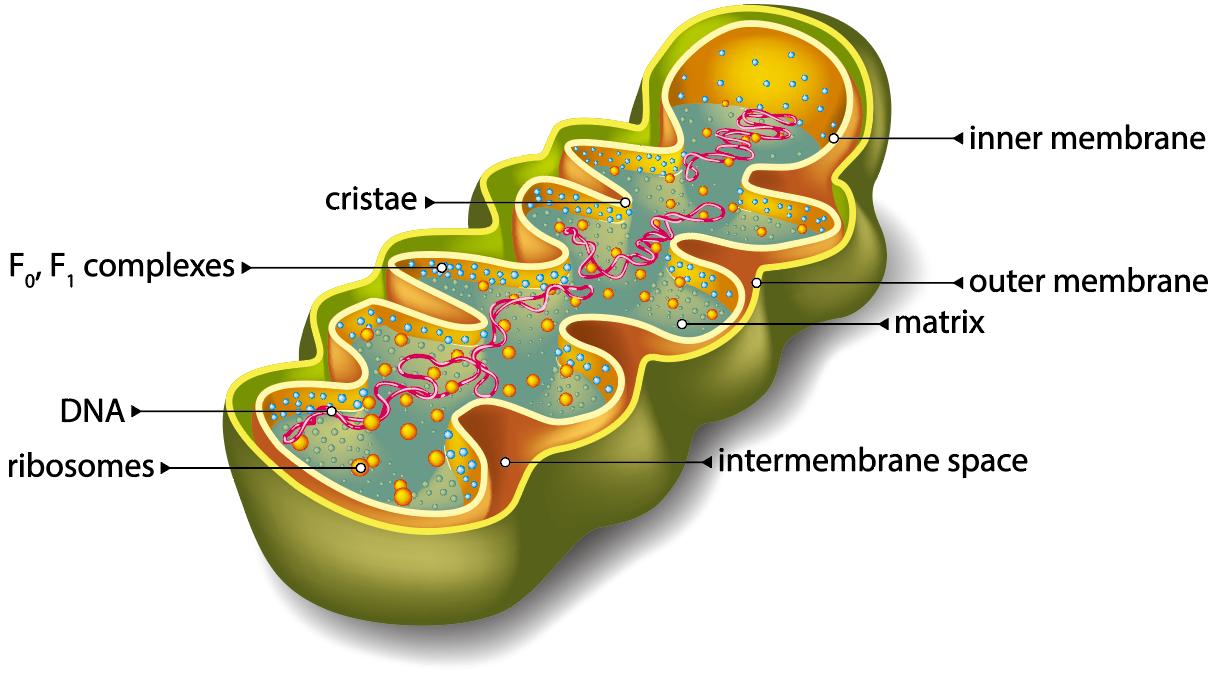 Structure of mitochondria
Structure of mitochondria
Preparation of Mitochondrial Protein Samples
Mitochondrial protein extraction from cell samples
1) Reagents should be prepared by pre-cooling and adding PMSF and sodium orthovanadate at a final concentration of 1 mM before use.
2) Cells should be collected at 4°C, centrifuged at 500g for 5 min, and washed several times with 0.25M buffered sucrose solution. Then homogenize the cells by adding 0.25M buffered sucrose solution at a ratio of 2x107 cells to 2mL at 0-4°C. The sucrose solution should be added in several times.
3) Add 2 mL of 0.34 M buffered sucrose solution to the bottom of the centrifuge tube first. Then carefully add 2mL of homogenate along the wall of the tube to cover the upper layer and centrifuge at 700g for 10min to collect the supernatant and precipitate separately.
4) The collected precipitate should be washed twice with 2mL of 0.25M sucrose solution, and the supernatant should be collected by centrifugation at 1000g for 15min each time.
5) The supernatant should be combined with 10000g and centrifuged for 10min to collect the precipitate.
6) Wash the collected precipitate with 2mL of 0.25M sucrose solution twice, centrifuge at 10000g for 15min each time, and collect the precipitate.
7) The precipitate was added to RIPA lysate (100uL of lysate for 1x106 cells), mixed with a gun, and placed on ice for 30min.
8) The lysed protein samples were sonicated on ice to make them more fully lysed and stored at -80°C.
Mitochondrial protein extraction from tissue samples
1) Reagents should be prepared by pre-cooling the reagents before use and adding PMSF and sodium orthovanadate at a final concentration of 1 mM.
2) Two clean dishes should be washed in 0.25 M buffered sucrose solution to remove impurities such as fat and blood from the tissue blocks. Absorb the water with filter paper, weigh and put them into a pre-cooled ceramic mortar and cut them into pieces. Then homogenize the tissue by adding 9 mL of cold 0.25 M buffered sucrose solution per gram of tissue at 0 to 4°C. The sucrose solution should be added in several portions.
3) Add 9 mL of 0.34 M buffered sucrose solution to the bottom of the centrifuge tube. Then carefully add 9 mL of homogenate along the wall of the tube to cover the upper layer. 700 g centrifuge for 10 min and collect the supernatant and precipitate separately.
4) The collected precipitate is then added to 10mL of pre-cooled 0.25M buffered sucrose solution twice, and the supernatant is collected by centrifugation at 1000g for 15min each time.
5) The supernatant should be combined with 10000g and centrifuged for 10min to collect the precipitate.
6) Add 10mL of pre-cooled 0.25M sucrose solution twice and centrifuge at 10000g for 15min each time to collect the precipitate.
7) Add the precipitate to RIPA lysate (100uL lysate for 1x106 cells), mix well with a gun and place on ice for 30min.
8) The lysed protein samples should be sonicated on ice to make them more fully lysed and stored at -80°C.
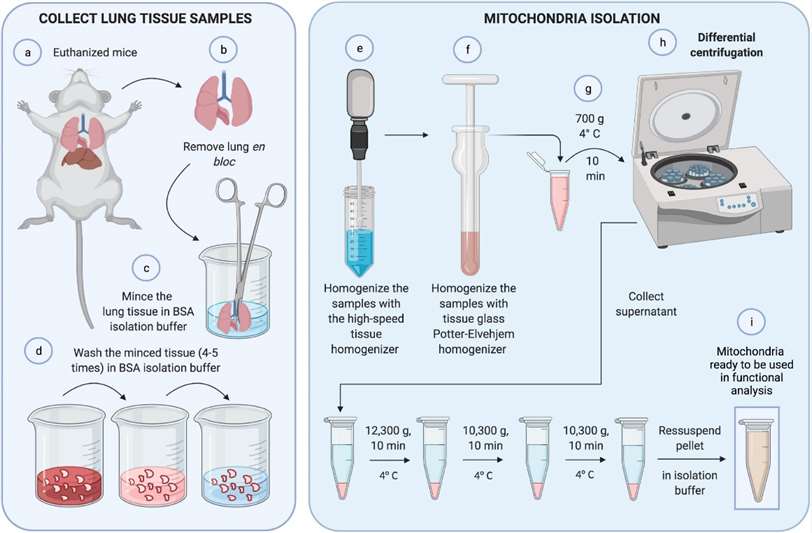 Mitochondrial isolation (Caldeira et al., 2021)
Mitochondrial isolation (Caldeira et al., 2021)
Reference
- Caldeira, D. D. A. F., Oliveira, D. F. D., et al. (2021). Isolation of Mitochondria From Fresh Mice Lung Tissue. Frontiers in Physiology, 2095.
* For Research Use Only. Not for use in diagnostic procedures.